Introduction
Searching for nothing would seem to be an absolute waste of time - unless you are responsible for cleaning validation. In that case, success is deliciously ironic since you can say, "I found nothing and that's good news."
But perhaps we should start at the beginning.
Those involved in the manufacture and quality control of pharmaceuticals and biotechnology products are aware of the need to prove that the production equipment and the manufacturing environment are sufficiently clean such that the next lot of product will not be contaminated by materials from the previous lot, i.e. that cross contamination will not be an issue. The procedures and documentation for that proof constitute what is known as cleaning validation. Essentially, one wants to prove with a high degree of certainty, that any residues of drug product from a manufacturing lot and any residues of cleaning agents used to remove those drug residues, are below acceptable limits. To put a fine point on it, we're not exactly searching for nothing (as in a zero value), but for extremely low values of residues - both drug product residues and cleaning agent residues.
Cleaning Validation
Cleaning validation can be considered a three step process, involving (i) the cleaning and rinsing of the requisite surfaces, (ii) sampling any drug or cleaning agent residues that might still remain on those surfaces and (iii) analysing the sampled materials with the appropriate instrumentation. Steps (i) and (ii) are manual, sometimes tedious procedures, but only if they are done properly do they permit accurate results to be reported in step (iii). The cleaning of surfaces (what cleaning agents to use, how to do the cleaning, etc.) and the analysis of the sampled materials (calibration curves, limits of detection) are themselves fascinating topics (at least to some people), but they are not our focus here. For this discussion, we will look only at step (ii) - the procedures that enable one to sample a cleaned surface with a high degree of reproducibility, to ensure that what is reported in step (iii) truly represents the condition of the sampled surface.
It may seem intuitively obvious, but one does not begin the cleaning validation process until there is an absence of visible residue on the surface. Residues are visible at surface concentrations of between 1 and 4 ug/cm2. The simple rule is that if you can still see residues on the surface, forget about any sampling activities, you haven't finished step (i) - the cleaning activities.
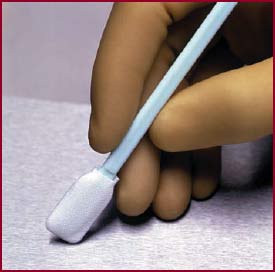 | Figure 1. A polyester knit swab used for surface sampling. |
Sampling Surfaces with Swabs
Assuming the surface is free of visible residue (i.e. that the cleaning stage is done), the challenge is now to sample that surface in a reproducible manner, such that any (invisible) residues, present in extremely small amounts, are collected and delivered to the instrument for measurement. The best type of swab for sampling is one with a head made of laundered polyester knit fabric (Figure 1), since that material provides the lowest levels of releasable particles, the highest recovery and the lowest background when total organic carbon (TOC) measurements are employed as the analytical technique. To sample the surface, the swab is moistened then drawn across the surface in a thorough and reproducible manner to collect any residue into the interstices of the polyester knit fabric. The swab is then deposited into a suitable collection vial, then the residues extracted from the swab head for subsequent analysis.
It is worth devoting a moment to the technique for moistening a swab, since errors in technique here will lead to inconsistent results. There might be a temptation to simply saturate the swab head with high-quality (e.g. TOC-grade) water to do the residue collection. This will cause problems, since the excess liquid on the swab head will simply spread the residue over the surface to be sampled and will not allow the residue to be picked up reproducibly into the swab fabric. For best results, the swab should be damp, but not saturated. This is best accomplished by immersing the head into a container of high-quality water, and pressing both sides of the swab head against the side of the container a few times to expel any air trapped in the fabric and allow the water to fully penetrate the fabric. Then the swab head is raised out of the water and the flat sides of the swab are drawn across the rim of the container to expel excess water and leave the swab head moist. The degree of moistness of the swab head (otherwise known as the percent wetting level) need not be identical from run to run, since residues will be picked up over a fairly wide range of swab head moistness.
 | Figure 2. A template for surface sampling. |
Ideally, to maximize reproducibility in swab head moistness, one would like to be able to dispense the optimum amount of water onto the swab head from a micropipet to produce, say, a 50% wetting level, but unfortunately this is impractical. The swab head material is not totally hydrophilic and water dispensed in such a manner will simply bead up on the swab head and will not be absorbed by the fabric.
 | Figure 3. Swabbing patterns |
 | Figure 4. A notched swab handle simplifies separating the swab head from the handle. Only the head needs to be extracted for TOC measurement. |
The manner in which the swab is used to sample the surface, i.e. the swabbing pattern, is critical to ensure accurate and reproducible collection of residues. For easily accessible surfaces, a template with a 5 cm x 5 cm opening can be used to sample the same surface area each time (Figure 2). As with wiping, linear overlapping strokes over the surface to be sampled will ensure that the residue is collected into the moist swab head. Figure 3 shows a typical sampling pattern employing 2 swabs. The first side of the first swab is swiped horizontally 10 times over the template opening, then the swab is flipped over and the second side is swiped vertically 10 times over the same surface. This swab is deposited into the collection vial (Figure 4). The first side of the second swab is swiped diagonally upwards 10 times, then flipped over and the second side swiped diagonally downward 10 times. The second swab is deposited into the same collection vial. In this manner, the surface has been swabbed a total of 40 times, and there is a reasonable expectation that any residue on the surface has been transferred into the two swab heads. It is not required to use two swabs; often one will do.
Operators can verify that their technique for dampening the swab heads and swabbing surfaces is appropriate through replicate recovery experiments of known challenges dispensed onto sample surfaces. Ideally, one would like to recover 100% of the challenge, but recoveries may be limited to 75%-80%, depending on the sampling conditions and the residue. It must be recognized that the swab head may not get everything off the surface and that the extraction liquid may not get all of the residue out of the swab head. Indeed, a 90% efficiency at each stage, produces an overall recovery of only 81% (i.e. 0.9 x 0.9).
Pre-cleaned vials and swabs are available commercially that provide TOC background levels of <10 ppb and <50 ppb TOC, respectively. This may be important if very low levels of residues are sought, since one never wants to report at an analytical value obtained by subtracting two large numbers to produce a small difference.
Conclusions
So the search for nothing really involves (i) good cleaning and rinsing protocols, (ii) proper swabbing techniques to ensure that any residues left on the surface are collected into the swab, and (iii) pre-cleaned vials and swabs for minimum background levels. Rigorous attention to detail and technique will enable success in finding nothing.






















